Transcranial Color Doppler in the Neuro-ICU
Published on: July 18, 2024
Introduction
Transcranial Color Doppler (TCCD) ultrasound is a versatile imaging modality with multiple potential uses in the acute setting. While computed tomography (CT) and magnetic resonance imaging (MRI) are typically the preferred imaging modalities in most situations, there are instances in which acute neurologic patients are too unstable to be transported to a scanner. In these cases, TCCD can serve as a useful adjunct to help guide clinical decisions.
Technique
Intracranial structures are best seen at a low frequency (2-2.5 Hz), utilizing the phased array probe and a cranial or abdominal preset on the ultrasound. The transtemporal window can be insonated to view intracranial structures, though it should be noted that about 10-20% of patients may not have adequate windows1. The operator can first identify the level of the midbrain and then proceed to fan up superiorly by ten degrees to the level of the third ventricle followed by the lateral ventricle2 (Figure 1). The depth should be adequate enough to visualize the contralateral skull. Given the nature of the artifact caused by the ipsilateral skull, the contralateral parenchyma is better visualized. Several structures can be identified by their hyperechoic nature, including the choroid plexus, pineal gland, and clinoid processes. Given that the choroid plexus and ependymal cells are hyperechoic, this can help identify the third and lateral ventricles. Conversely, the midbrain and thalami appear hypoechoic2.
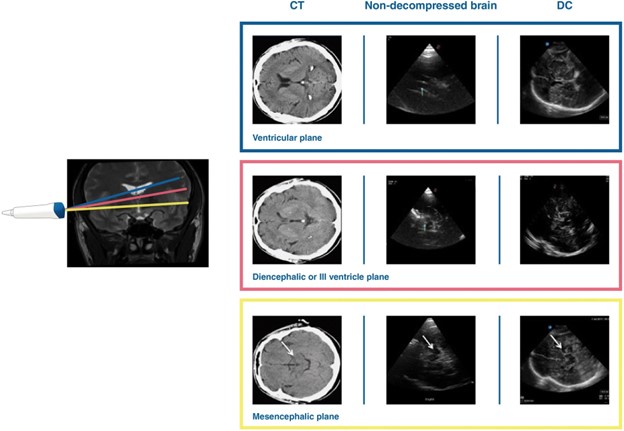
Figure 1. By fanning the probe superiorly by ten degrees each time2, insonating at the transtemporal window can show structures at three different levels: the mesencephalic plane, the plane of the third ventricle, and the plane of the lateral ventricle.
Clinical Applications
Midline shift can be measured utilizing TCCD. Distance can be measured from both the ipsilateral and contralateral skull to a midline structure, particularly the third ventricle, septum pellucidum, or pineal gland2. Detection of midline shift has been shown to correlate with CT imaging and can also predict outcomes, particularly in patients with malignant ischemic stroke3.
Intracerebral hemorrhage (ICH) can cause significant perihematomal edema, midline shift, and herniation. Acute blood within the brain parenchyma is hyperechoic and thus can be seen with TCCD. Blood on the contralateral side from the probe is seen best with this technique4 (Figure 2). The size of the ICH can be estimated utilizing measuring tools on the ultrasound, with measurements correlating to those obtained from CT imaging for hemorrhages larger than one centimeter5. However, convexity and infratentorial hemorrhages are not well visualized on TCCD, nor are subacute hemorrhages which are less hyperechoic, or extraparenchymal hemorrhages which are affected by skull artifact. In these situations, TCCD is therefore not ideal4,6,7.
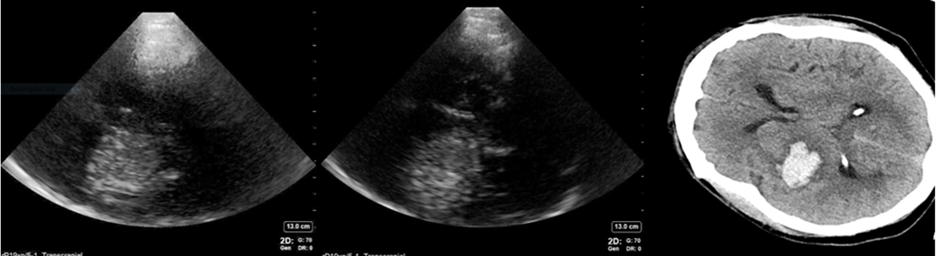
Figure 2: An example of TCCD showing an ICH contralateral to the side that is being insonated. The ICH appears hyperechoic on ultrasound. Images obtained and used with permission from Aarti Sarwal, MD.
Hydrocephalus can also be evaluated with TCCD in a patient who cannot travel for CT imaging. Note, however, that the trend of measurements of the third ventricle is more reliable than a single measurement given that size can vary from patient to patient6. In general, the third ventricle should measure between 2 and 10 mm, with measurements over 10 mm associated with hydrocephalus8,9 (Figure 3). In measuring the degree of hydrocephalus in patients undergoing clamp trials for extraventricular drain (EVD) weans, those with a 5.5 mm or greater increase in ventricle size were more likely to fail their wean10. Measurement of hydrocephalus at the cerebral aqueduct or lateral ventricles has much lower interrater reliability, thus the third ventricle is the preferred area to measure8,9.
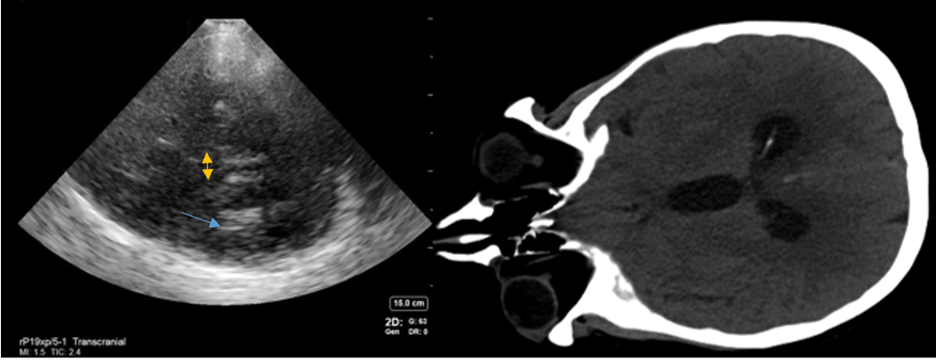
Figure 3: An example of how to measure hydrocephalus on TCCD. The yellow arrows show the third ventricle, while the blue shows the hyperechoic ependymal cells of the lateral ventricle on the side contralateral to the probe. Images obtained and used with permission from Aarti Sarwal, MD.
Post-neurosurgical patients, especially those with a decompressive hemicraniectomy, can be monitored with TCCD as well. When combined with clinical examination, this can help detect many post-surgical complications. Intraparenchymal structures are much clearer and detailed in those with a hemicraniectomy due to the lack of artifact from the ipsilateral skull11 (Figure 4). In hemicraniectomy patients, an abdominal preset should be used as opposed to the transcranial preset as the thermal indices are lower. As little pressure as possible should be applied on the probe to avoid damaging the dura or other underlying structures2. Presence of midline shift, ICH, or hydrocephalus can be easily visualized in this population and aid in clinical decision making.
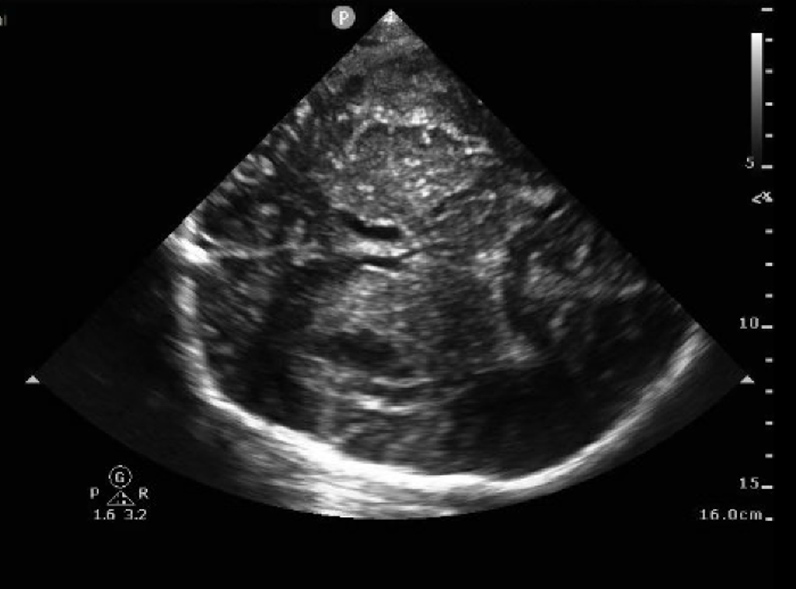
Figure 4. An example of TCCD in a patient with a hemicraniectomy. The brain parenchyma is seen with more detail than if the ipsilateral skull remained present. The lateral ventricles are easily identified as they are surrounded by hyperechoic ependymal cells. Images obtained and used with permission from Brittany Kasturiarachi, DO.
Conclusion
TCCD can be a valuable adjunctive tool in the intensive care unit to evaluate many different kinds of pathologies in the acute neurologic patient, especially when patients are too unstable to receive CT or MRI scans.
References
- He L, Wu D-F, Zhang J-H, Zheng S, Li Y, He W. Factors affecting transtemporal window quality in transcranial sonography. Brain Behav. 2022;12(4):e2543. doi:10.1002/brb3.2543
- Robba C, Goffi A, Geeraerts T, et al. Brain ultrasonography: methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med. 2019;45(7):913-927. doi:10.1007/s00134-019-05610-4
- Gerriets T, Stolz E, Modrau B, Fiss I, Seidel G, Kaps M. Sonographic monitoring of midline shift in hemispheric infarctions. Neurology. 1999;52(1):45-49. doi:10.1212/wnl.52.1.45
- Allen BC, Kapoor S, Anzalone A, et al. Transcranial ultrasonography to detect intracranial pathology: A systematic review and meta-analysis. J Neuroimaging. 2023;33(3):333-358. doi:10.1111/jon.13087
- Gómez-de Frutos MC, García-Suárez I, Laso-García F, et al. B-Mode Ultrasound, a Reliable Tool for Monitoring Experimental Intracerebral Hemorrhage. Front Neurol. 2021;12:771402. doi:10.3389/fneur.2021.771402
- Seidel G, Kaps M, Gerriets T, Hutzelmann A. Evaluation of the ventricular system in adults by transcranial duplex sonography. J Neuroimaging. 1995;5(2):105-108. doi:10.1111/jon199552105
- Dinsmore M, Venkatraghavan L. Clinical applications of point-of-care ultrasound in brain injury: a narrative review. Anaesthesia. 2022;77 Suppl 1:69-77. doi:10.1111/anae.15604
- Ryan S, McNicholas M, Eustace SJ. Anatomy for Diagnostic Imaging. 3rd, illustrated ed. Saunders/Elsevier; 2011.
- Ozdemir O, Calisaneller T, Hastürk A, Aydemir F, Caner H, Altinors N. Prognostic significance of third ventricle dilation in spontaneous intracerebral hemorrhage: a preliminary clinical study. Neurol Res. 2008;30(4):406-410. doi:10.1179/174313208X276240
- Kiphuth IC, Huttner HB, Struffert T, Schwab S, Köhrmann M. Sonographic monitoring of ventricle enlargement in posthemorrhagic hydrocephalus. Neurology. 2011;76(10):858-862. doi:10.1212/WNL.0b013e31820f2e0f
- Sarwal A, Elder NM. Point-of-care Cranial Ultrasound in a Hemicraniectomy Patient. CPCEM. 2018;2(4):375-377. doi:10.5811/cpcem.2018.7.39379
Author Affiliations
- Neurocritical Care Fellow; University of Cincinnati