The Role of Ultrasound Guided Lumbar Puncture in the Neuroscience Intensive Care Unit: A Review and Case Presentation
Published on: June 12, 2025
Introduction
Ultrasound-guided approaches to procedures such as lumbar punctures (LP) have demonstrated significant advantages over traditional palpation-based techniques in various clinical settings, including the neuroscience intensive care unit (Neuro ICU). Studies highlight several key benefits of ultrasound guidance, including improved success rates, reduced procedural time, fewer complications, and enhanced patient comfort, especially in patients with larger body habitus or anatomical variations. These advantages become indispensable in the Neuro ICU, where the window of opportunity to intervene on acute neurological diseases requires expedient analysis of cerebrospinal fluid.
Across a spectrum of experience and backgrounds, providers performing lumbar punctures succeed more frequently with ultrasound guidance. In the first meta-analysis comparing palpation versus ultrasound-guided techniques in 14 randomized controlled trials (of which 9 trials consisted of epidural catheterization), pooled data revealed that ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%–8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure.1 In a second meta-analysis using pooled studies of diagnostic lumbar puncture, spinal anesthesia, and epidural catheterization, there was a lower combined risk of technical failure (risk ratio 0.51, 95% CI, 0.32-0.80) with an ultrasound-guided technique compared to a palpation-based technique.2 In the most recent meta-analysis of only diagnostic lumbar puncture, Gottlieb et al. found higher success rates in the ultrasound-guided group than in the palpation-guided group (90% vs. 81%), with an odds ratio of 2.1 (95% CI 0.66-7.44). Their analysis also revealed ultrasound-guided LP was associated with fewer traumatic taps (10.7% vs. 26.5%) and reduced pain compared to palpation techniques (3.75 vs. 6.31).3 Similarly, a randomized controlled trial by Evans et al. noted a significant decrease in the number of needle insertion attempts when using ultrasound (1.54x more attempts, p = 0.046).4
Ultrasound-guided LP allows for the rapid identification of various anatomical structures relevant to the performance of an LP. In one cohort study, Ferre et al. found that high quality images of spinous processes, laminae, ligamentum flavum, dura mater, the epidural space, and the subarachnoid space could be obtained in around 1 minute (mean acquisition time 57.19 seconds; SD, 68.14 seconds; range, 10-300 seconds).5
As patients’ BMIs increase, identifying these structures to guide placement and trajectory of the needle becomes exceedingly helpful. When comparing a palpation-based technique to an ultrasound-guided technique in patients with variable body mass index (BMI), Stiffer et al. found that success rates in identifying landmarks using palpation correlated with BMI. There was difficulty palpating landmarks in 5% of patients with normal BMI, 33% in patients who were overweight, and 68% of patients who were obese (p < 0.0001). In those patients whose landmarks were difficult to palpate, ultrasound allowed for the identification of relevant anatomy in 16 of 21 patients (76%)6. To illustrate how we use ultrasound at our institution, we will outline our ultrasound procedure and some typical cases we encounter within our Neuro ICU.
Technique
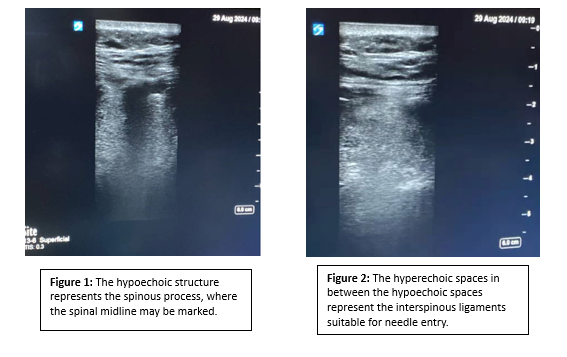
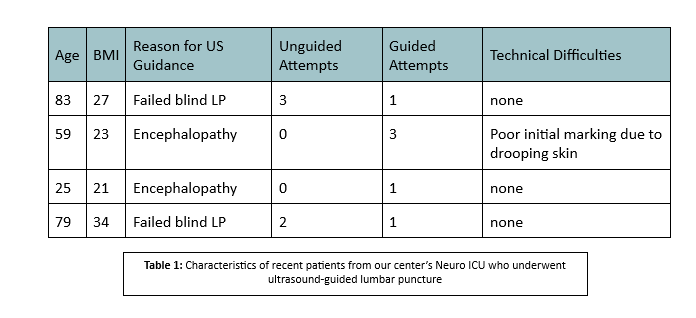
We use a high-frequency linear probe for greater resolution in order to identify anatomical landmarks. These include the spinous process on a transverse plane (the lumbar spinous process is centered on the screen, and a mark is made perpendicular to the transducer) and the interspinous ligament on a longitudinal plane (a mark is again made perpendicular to the transducer). Where the two lines intersect marks the ideal target for spinal needle insertion. A curvilinear or low-frequency phased-array probe may be used if a greater depth is desired, though this greater depth comes at the expense of reduced resolution. To orient ourselves to the patient’s vertebral levels, we begin by identifying the sacrum, whose appearance is distinct, and proceed to mark the two levels above that as possible insertion sites.
Case 1
An 83-year-old white female with a recent history of upper respiratory tract viral illness was brought into the emergency department after being found by a neighbor to be lethargic, confused, and with evidence that she had urinated herself. Upon arrival she was found to be febrile with a neurological exam revealing aphasia, right gaze preference, left facial droop, and left hemiparesis. Point of care EEG revealed status epilepticus. The patient was subsequently loaded with Ativan and Keppra and transferred to the Neuro ICU for further management. Overnight, a blind lumbar puncture was unsuccessful. An ultrasound guided LP was subsequently performed successfully.
A lumbar puncture revealed predominantly lymphocytic pleocytosis with 12/uL nucleated cells and 82 mg/dL protein level. She was subsequently continued on IV acyclovir given the CSF evidence of a likely viral or tick-borne encephalitis.
Case 2
A 59-year-old female with a history of Stage 4 small cell lung cancer with extensive metastases presented with one week of confusion, staring spells, and intermittent aphasia. The patient also had a recent hospitalization of grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS). The patient was found to be disoriented, inattentive, and lethargic without evidence of focal deficits. An MRI brain did not reveal any abnormalities. Given the patient’s encephalopathy and poor cooperation, we opted for an ultrasound-guided lumbar puncture to reduce the number of punctures and avoid the use of sedating medications. An ultrasound guided lumbar puncture was subsequently performed revealing an elevated CSF protein of 109 mg/dL with minimal pleocytosis. Subsequent CSF analysis ruled out infectious etiologies and the patient was started on dexamethasone 10 mg every 6 hours for treatment of recurrent Grade 3 ICANS.
Conclusion
In conclusion, ultrasound-guided lumbar puncture offers considerable advantages over traditional techniques, including higher success rates, increased efficiency, and more patient comfort, especially in patients with higher BMIs and abnormal anatomy. These benefits make ultrasound guidance useful for LPs in the neuro ICU, where precision, speed, and patient comfort define the standard of care.
References
1. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. doi: 10.1136/bmj.f1720.
2. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251–260. doi: 10.1097/AAP.0000000000000184.
3. Gottlieb, M., Holladay, D., & Peksa, G. D. (2019). Ultrasound‐assisted lumbar punctures: a systematic review and meta‐analysis. Academic Emergency Medicine, 26(1), 85-96.
4. Evans, D. P., Tozer, J., Joyce, M., & Vitto, M. J. (2019). Comparison of ultrasound‐guided and landmark‐based lumbar punctures in inexperienced resident physicians. Journal of Ultrasound in Medicine, 38(3), 613-620.
5. Ferre, R. M., & Sweeney, T. W. (2007). Emergency physicians can easily obtain ultrasound images of anatomical landmarks relevant to lumbar puncture. The American journal of emergency medicine, 25(3), 291-296.
6. Stiffler, K. A., Jwayyed, S., Wilber, S. T., & Robinson, A. (2007). The use of ultrasound to identify pertinent landmarks for lumbar puncture. The American journal of emergency medicine, 25(3), 331-334.