The Role of Nurses in Managing Severe Hyponatremia and Preventing Central Pontine Myelinolysis
Published on: January 09, 2025
Introduction
The role of nurses in monitoring patients’ clinical condition and laboratory results is critical in preventing complications from overly rapid correction of hyponatremia. This article discusses the unfortunate case of a young patient who developed central pontine myelinolysis (CPM) after overly rapid correction of his serum sodium level and how nursing care should address potential issues in the management of hyponatremic patients long before complications arise. CPM is a syndrome of non-inflammatory etiology that causes damage to the myelin sheath. Demyelination tends to occur symmetrically and most frequently within the central pons, but may also occur within the basal ganglia, thalamus, midbrain, and cerebellum as part of the broader osmotic demyelination syndrome (ODS).
Rapid correction of prolonged hyponatremia creates osmotic fluctuation leading to cellular edema. However, the exact nature of the precipitating factor leading to demyelination is poorly understood. In addition to hyponatremia, CPM has been associated with alcoholism, malnutrition, and hepatic disease3. There is no standardized treatment, though efforts including courses of intravenous immunoglobulin (IVIg) or plasmapheresis have been described to counteract the potential contributory effects of circulating autoantibodies. It is unclear what degree of sodium correction may induce CPM, but it is commonly suggested to correct no more than 10 mmol/L per 24-hour period and no more than 18 mmol/L in 48 hours. Additionally, the magnitude of correction may be of more clinical significance than rate alone5. Potential symptoms associated with CPM include quadriparesis, bulbar dysfunction, “locked-in” syndrome, facial palsy, nystagmus, coma, and ataxia3.
Role of the Registered Nurse
The development of neurologic deficits in patients with suspected CPM is a very late manifestation. Nevertheless, nurses should perform regular neurologic assessments on patients admitted with hyponatremia, even if the admission assessment reveals no evidence of neurologic injury. Nurses should assess for changes in mental status, weakness, dysphagia, dysarthria, or ocular findings and report these concerns expeditiously. Critically, nurses should carefully monitor the rate at which the sodium level for patients with hyponatremia is corrected. If serial sodium levels or basic metabolic profile testing are not already ordered, the nurse should contact the medical team and advocate for this testing to be done regularly, at least every six hours, especially if more aggressive treatment such as hypertonic saline or aquaretics (i.e., vasopressin receptor antagonists) is implemented. The nurse should also request a sodium parameter, so that all members of the patient’s care team are aware of the goal rate at which to correct the sodium level within a 24-hour period (ideally, not more than 8-12 mEq/L per day). In this way, correction rates which are too fast can be promptly addressed.
An essential part of the nursing assessment should also include a review of home medications. Select medications, including some antidepressants and anticonvulsants, are known to cause hyponatremia. If the use of these medications is discovered during the admission assessment of patients’ home medications, nurses should bring this to the attention of medical staff. For patients with critically low sodium levels, nurses should pre-emptively place patients on seizure precautions and monitor for any clinical evidence of possible seizure. Patients with severe electrolyte derangements may also be confused, and ongoing nursing care should be targeted towards the prevention of delirium and regularly re-orienting the patient.
Patient Case
A 21-year-old man with Meckel’s diverticulum underwent small bowel resection and lysis of adhesions and was subsequently discharged home. He presented to the hospital again approximately two weeks later with nausea, vomiting, and abdominal pain, and on admission was found to have a sodium level of 96 mmol/L. He was noted to be mildly lethargic but easily arousable to voice, followed all commands, and had no focal deficits. He was on no diuretics or other medications.
Initial laboratory investigations revealed a sodium of 96 mEq/L and chloride of less than 60 mEq/L, with other electrolytes generally within normal ranges. A CT head was not obtained on the day of admission, but a CT abdomen and pelvis with oral and IV contrast showed marked small bowel obstruction. Because of the severe hyponatremia and lethargy, the patient was admitted to surgical critical care and the bowel obstruction was initially managed medically. Correction of the hyponatremia was instituted.
The sodium was corrected to 126 in a 24-hour period, ultimately reaching a peak of 132. Over the course of three days, progressive neurologic decline was noted, including increasing lethargy and possible seizure activity. Admission to Neurocritical Care was requested on hospital day 7, as neurologic examination was now consistent with locked-in syndrome and the patient required intubation—aside from eye blinking, facial movements were absent and the patient was otherwise quadriparetic. No meaningful interaction was ascertainable with eye movements. Sodium on admission to the Neuro ICU was 132. A brain MRI confirmed a diagnosis of CPM, showing central pontine high intensity signal sparing the peripheral regions, as well as symmetric high intensity signal in the bilateral basal ganglia.
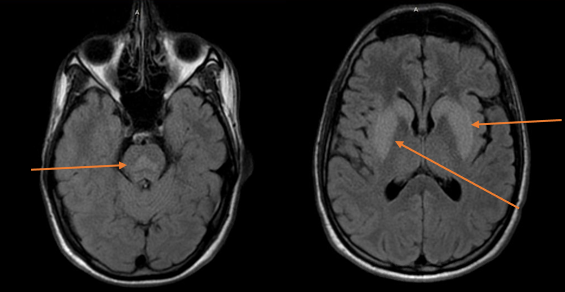
Figure 1. Brain MRI showing high intensity signal in the central pons, and symmetric high intensity signal in the bilateral basal ganglia (arrows) suggesting central pontine myelinolysis.
In response to these findings, the patient’s sodium was lowered to a goal of 120-125 mEq/L with free water flushes and intravenous hypotonic fluids. These parameters were maintained for the first 10 days of his neurocritical care course, after which the enteral water and intravenous hypotonic fluids were gradually withdrawn and the patient’s sodium was allowed to slowly increase in small increments over the next three days until a sodium of 135-140 mEq/L was reached.
In parallel, the patient was started on a five-day course of IVIg at a dose of 400 mg/kg daily, after which there was no significant clinical improvement immediately noted aside from purposeful eye-tracking and following commands to open and close his eyes. This was followed by a six-day course of plasmapheresis, which the patient’s family was initially resistant to starting because they wanted to avoid additional procedures such as placement of a dialysis catheter. However, on the fourth day of plasmapheresis, the patient was noted to have improved significantly, having started to be able to vocalize and move his extremities against resistance. By day 7 in the neuro ICU, he had improved to the point of being able to ambulate with assistance, and he was noted to be cognitively intact. He continued to progress clinically, and plasmapheresis was continued twice weekly for three weeks and then once weekly for three additional weeks. At the end of his hospitalization, the patient was able to ambulate with minimal assistance and perform activities of daily living, and he was discharged to acute rehabilitation for further treatment. By the time of outpatient follow-up, the patient was able to run normally. About four months after onset of CPM, neuropsychological testing revealed appropriate orientation, mild impulsivity, and intermittent mild word finding difficulty, with a favorable prognosis for recovery and no further neuropsychological evaluations or interventions planned.
Discussion
Extreme caution must be taken in correct serum sodium levels in hyponatremic patients, particularly when there should be a higher index of suspicion for a more chronic nature. Though commonly thought of as a neurologic disorder limited to this scenario, it has also been described in patients with acute hypernatremia and overly rapid lowering of the serum sodium level.
In patients who develop CPM and receive treatment with IVIg or plasmapheresis, nurses should be aware of several potential issues related to these treatments. Because IVIg may predispose patients to an elevated risk of thrombosis, nurses should monitor for signs of venous thromboembolism, prophylactically apply sequential compression devices, and advocate for pharmacologic prophylaxis when appropriate. Renal function should also be monitored closely for the duration of therapy and thereafter, with especially close monitoring by nursing staff recommended during initiation of therapy.
Plasmapheresis also poses its own challenges. Because plasmapheresis may use either 5% albumin or fresh frozen plasma, nurses should ensure an active type and screen is always maintained and that patients are aware of and willing to undergo the procedure (e.g., Jehovah’s Witnesses who refuse blood products). Further, because plasmapheresis may require the insertion of a central venous catheter at many institutions, there are associated risks of infection, pneumothorax, pain, and vascular injury that nurses should monitor for. Nurses should also be aware of risks associated with treatment with plasmapheresis, including bleeding (especially when albumin is used as the replacement product, since it can lead to lower fibrinogen levels), transfusion reactions, and hypocalcemia, all of which should be monitored closely.
References
- Chang, K., Lee, I., Kim, G., Cho, K., Park, H., & Kim, H. (2014). Plasmapheresis successfully treats central pontine myelinolysis after acute hypernatremia from intravenous sodium bicarbonate therapy. BMC Nephrology, 15(56), 1-5.
-
Hegazi, M., & Nawara, A. (2016). Prevention and treatment of osmotic demyelination syndrome: A review. JSM Brain Science, 1(1), 1-7.
-
Rebedew, D. (2016). Is central pontine myelinolysis reversible? Wisconsin Medical Journal, 115(6), 326-328.
-
Soupart, A., Pennickx, R., Stenuit, A., Perier, O., & Decaux, G. (1996). Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. Journal of Neuropathology and Experimental Neurology, 55, 594-601. doi:10.1097/00005072-199605000-00011
-
Sterns, R., Riggs, J., & Schochet, S. (1986). Osmotic demyelination syndrome following correction of hyponatremia. New England Journal of Medicine, 314, 1535-1542. doi:10.1056/NEJM198606123142402