Cefepime’s Double-Edged Sword: Neurotoxicity in Critically Ill Patients
Published on: July 24, 2024
As a fourth-generation anti-Pseudomonal cephalosporin, cefepime is one of the first-line antimicrobials used to treat various hospital-associated bacterial infections. Similar to other beta-lactams, it is generally well-tolerated. In the setting of critical illness, cefepime is recommended to be dosed at 2 g every 8 hours to optimize pharmacodynamic target attainment (time over minimum inhibitory concentration, MIC), particularly in infections caused by organisms with higher MIC.1 Cefepime extended infusions are recommended to overcome variable serum concentrations in patients with obesity2 or augmented renal clearance3, such as those of younger age, post-trauma or burn, or with sepsis, and have become increasingly popular for use in other critically ill patients as well. As cefepime is primarily excreted via the urine as unchanged drug, it requires renal dose adjustments in patients with renal insufficiency, particularly in elderly patients (Table 1). These occur at relatively higher thresholds than other beta-lactams, requiring adjustment once creatinine clearance falls below 60 mL/min, which is not uncommon in acute kidney injury or older age.
Table 1. Cefepime renal dose adjustments recommended by manufacturer.4
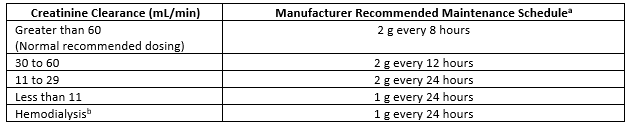
aMaintenance regimens for cefepime extended infusions utilize the same dosing adjustments, infused over 4 hours with a bolus loading dose.
bAdminister following hemodialysis on hemodialysis days.
Failure to dose reduce in renal insufficiency can result in encephalopathy, myoclonus, and seizures.4 Cefepime-induced neurotoxicity is associated with increased morbidity and mortality, though its correlation with in-hospital mortality is difficult to separate from other causes in critical illness.5 In 2012, the U.S. Food and Drug Administration released a warning following the report of several cases of non-convulsive status epilepticus associated with cefepime.6
Incidence of cefepime-induced neurotoxicity varies widely, ranging from 1% to up to 24% of ICU patients.5,7-9, 11 Cases are predominantly reported in patients who are elderly, have renal dysfunction, and/or require intensive care.5,9-12 In the ACORN trial—the only randomized trial to evaluate cefepime-induced neurotoxicity to date—cefepime was associated with higher rates of encephalopathy, with 21% fewer delirium- and coma-free days compared to piperacillin-tazobactam in ED and medical ICU patients.11 Although the incidence is higher in patients who did not receive appropriate renal dose reductions, Fugate et al. reported that 29% of ICU patients still experienced neurotoxicity despite receiving dosing appropriate for their renal function.7 Similarly, Payne et al. reported that over a quarter of patients with cefepime-induced neurotoxicity received appropriate dosing for their renal function; however, serum concentrations obtained in seven of these patients were elevated (≥20 mg/L).10 It is important to note that although 44% of the patients evaluated in this study were identified to be critically ill at the time of cefepime administration, it is unclear if the patients developing neurotoxicity at renally-appropriate doses were those in the ICU.
Risk of cefepime-induced neurotoxicity in patients receiving cefepime extended infusion remains largely unknown. A retrospective study by Venugopalan et al. found no significant difference in the incidence of neurotoxicity in 70 patients who received cefepime extended infusions.5
Patients with pre-existing neurological or neurovascular conditions—particularly those in the neurocritical care unit—are not well represented in the existing literature that evaluates cefepime-induced neurotoxicity. Most studies do not elucidate if the patients were located in the neurocritical care unit when receiving cefepime. Case reports primarily describe patients in the ICU for septic shock or post-operatively, though Fugate et al. identified one patient in the neuroscience ICU.7 Less than 20% of patients reported with neurotoxicity are described to have pre-existing CNS disease, including cerebrovascular disease, seizures, intracranial hemorrhage, encephalopathy, encephalitis, cerebral palsy, or dementia.5,9,10 However, in the few cases described among patients with stroke or pre-existing seizures, there was no significant difference in the incidence of neurotoxicity.5
Cefepime-induced neurotoxicity typically presents as confusion, diminished level of consciousness, and other symptoms of encephalopathy.8-13 Less commonly, patients demonstrate agitation and aphasia. Symptoms may progress to myoclonus and seizures, with non-convulsive seizures being more common than generalized convulsions.5,12 Typical EEG findings of cefepime-related encephalopathy show diffuse background slowing in the delta and theta ranges and generalized periodic triphasic discharges.5,14-15 Symptom onset is generally reported between 2 to 6 days following the start of drug therapy, although there is a considerable time lag between onset and diagnosis of cefepime-induced neurotoxicity. Most patients have partial to complete resolution of symptoms approximately 24 to 48 hours after cefepime discontinuation.
The exact mechanism of cefepime-induced neurotoxicity is not fully understood. Cefepime has been demonstrated to cross the blood brain barrier in the setting of inflammation, reaching CSF concentrations of 5 to 58% of serum concentrations.16 Active transport of cefepime out of the CSF to the blood may be impaired in renal dysfunction and critical illness due to the accumulation of toxic organic acids and decreased protein binding.10,14-15 The commonly accepted mechanism is that cefepime inhibits GABA-related inhibitory activity through competitive antagonism at GABA-A receptors in a concentration-dependent fashion, thus resulting in central excitotoxicity.5,8,10,17 Fernández-Fernández and Ameneiros-Lago proposed an additional mechanism through cefepime-related hypocarnitinemia, due to cefepime’s inhibition of OCTN2-mediated carnitine transport resulting in urinary loss of carnitine.18 Since hypocarnitinemia results in impaired mitochondrial fatty acid oxidation and has been implicated in neurotoxicity secondary to valproic acid toxicity, the authors hypothesized the potential contribution of carnitine deficiency in the setting of cefepime-induced neurotoxicity.
Management of Cefepime-Induced Neurotoxicity
Management of cefepime-induced neurotoxicity primarily consists of prevention, rapid recognition and diagnosis, and withdrawal of the offending agent. As cefepime-induced neurotoxicity is primarily reported in patients with renal insufficiency or age-related changes in renal function, emphasis is placed on dose reductions based on the patient’s estimated renal function. However, highly variable renal function and frequent concurrent nephrotoxic medications, particularly in critically ill patients, often results in the inability to detect renal insufficiency in a timely fashion. Dosing recommendations rely on creatinine clearance estimates, which may be delayed when creatinine-based equations are used in the setting of acute changes in renal function.10 In patients with advanced chronic kidney disease, serum creatinine and estimated creatinine clearance do not always correlate with true renal function.7 As such, these patients may receive doses adjusted for their calculated creatinine clearance, but not appropriate for their true renal function, as demonstrated by serum cefepime levels obtained in Payne et al.10
Cystatin C has been proposed as an alternative marker for renal function as it is not affected by muscle mass and has been found to have a stronger association with estimated glomerular filtration rate.19 KDIGO recommends the use of equations that combine both creatinine and cystatin C when available for drug-related decision making in patients with chronic kidney disease 20; however, there is limited data on the use of cystatin C to guide renal dose adjustment of cefepime. Kim et al. found that cystatin C-guided dose adjustment was associated with an 89% decreased risk of cefepime-induced encephalopathy compared to dose adjustments based on serum creatinine.21
Furthermore, cefepime-induced neurotoxicity has been reported despite concurrent hemodialysis, even with appropriate dose adjustments.7,9 Cefepime dosing in continuous renal replacement therapy (CRRT) highlights the difficult balance between adequate doses for antimicrobial effects and avoiding elevated serum concentrations placing patients at risk of cefepime-induced neurotoxicity. Recommended dose adjustment for cefepime in CRRT equates doses used in patients with creatinine clearance of approximately 30 to 60 mL/min (e.g., 2 g every 12 hours for Pseudomonal infections). Venugopalan et al. found that CRRT was independently associated with development of cefepime-induced neurotoxicity.5 In contrast, Honore and Spapen recommend against overly cautious dose reductions as patients receiving recommended CRRT dosing only obtain approximately 90% coverage of organisms with minimum inhibitory concentrations ≤2 µg/mL.22
Therapeutic drug monitoring (TDM) has been explored as a tool for identifying patients receiving supratherapeutic concentrations and at risk of adverse events. While TDM has been widely used for antimicrobials medications with narrow therapeutic windows, namely vancomycin and aminoglycosides, its use in cephalosporins is still limited. Widespread adoption has been limited by the assays’ unavailability, as well as the lack of well-defined therapeutic thresholds. Several retrospective studies evaluating TDM for other beta-lactams have found poor correlation between high serum concentrations and rates of various toxicities.23 Similarly, the exact concentration threshold for neurotoxicity has not been well elucidated.5 Boschung-Pasquier et al. found that a median trough concentration of 21.6 mg/L (IQR 17.0-28.6 mg/L) was associated with neurotoxicity.13 In contrast, Huwyler et al. and Venugopalan et al. found toxicity to be associated with much higher troughs, averaging 52.2 mg/L and 61.8 mg/L, respectively.5,24
Since cefepime-induced neurotoxicity is highly reversible, timely recognition and withdrawal of cefepime is important in reducing morbidity. Continuous EEG monitoring in patients with altered mental status and encephalopathy can be useful in providing diagnostic clarity and identifying non-convulsive status epilepticus.17 However, this degree of monitoring is not often available or feasible for most patients without other neurological conditions, even at resource-rich institutions. In addition, some providers argue that EEG abnormalities may not be specific to cefepime-induced neurotoxicity and instead reflect underlying metabolic changes, such as the triphasic waves seen in uremic or hepatic encephalopathy.17 In patients found to be in non-convulsive status epilepticus, treatment can include administration of anti-seizure medications. Since cefepime-related neurotoxicity typically resolves with simple cessation of the drug, these patients are unlikely to need long-term anti-seizure medications.8,17
In my practice, providers in the neurocritical care unit generally avoid the use of cefepime due to the paucity of literature in this population. For empiric or targeted coverage of Pseudomonas spp., alternative agents such as piperacillin-tazobactam and meropenem are considered, balancing the poor CNS penetration of the former antimicrobial with stewardship of the latter. I encourage deeper discussions of the risks of neurotoxicity in patients with multidrug-resistant Pseudomonas, noting that cefepime remains an optimal antimicrobial when resistance mechanisms to meropenem—primarily through drug efflux via porins—are present. Furthermore, alternative antimicrobials such as ceftazidime, piperacillin-tazobactam, carbapenems, and fluoroquinolones are each associated with their own risk of neurotoxicity, including seizures, with limited data comparing the rates of neurotoxicity with cefepime. In contrast, providers in our medical, surgical, and cardiac ICUs continue to use cefepime as a first-line agent with careful and frequent monitoring of and dose adjustments for renal function. Cefepime-induced neurotoxicity is often hard to identify in practice, due to the many confounding causes of altered mental status. However, if neurotoxicity is suspected, cefepime is immediately changed to an alternative antimicrobial.
Given the frequent use of cefepime for healthcare-associated infections, it is vital that clinicians are mindful of cefepime-induced neurotoxicity. Cautious and frequent dosing adjustments should be prioritized in renal insufficiency to mitigate the risk. The use of TDM should be further investigated to determine optimal therapeutic windows to ensure adequate exposure while minimizing toxicity. While it is not necessary to avoid the use of cefepime entirely, there are select patient populations – for example, elderly patients and those with renal dysfunction – in which cefepime should be considered with caution and alternative antimicrobial options considered.
References
- Kassel LE, Van Matre ET, Foster CJ, et al. A Randomized Pharmacokinetic and Pharmacodynamic Evaluation of Every 8-Hour and 12-Hour Dosing Strategies of Vancomycin and Cefepime in Neurocritically ill Patients. Pharmacotherapy. 2018;38(9):921-934. doi:10.1002/phar.2156
- Meng L, Mui E, Ha DR, Stave C, Deresinski SC, Holubar M. Comprehensive guidance for antibiotic dosing in obese adults: 2022 update. Pharmacotherapy. 2023 Mar;43(3):226-246. doi: 10.1002/phar.2769
- Silva CM, Baptista JP, Santos I, Martins P. Recommended Antibiotic Dosage Regimens in Critically Ill Patients with Augmented Renal Clearance: A Systematic Review. Int J Antimicrob Agents. 2022 May;59(5):106569. doi: 10.1016/j.ijantimicag.2022.106569
- MAXIPIME (Cefepime Hydrochloride, USP) [package insert]. Lake Forest, IL: Hospira, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf. Published June 2012. Accessed August 17, 2023.
- Venugopalan V, Casaus D, Kainz L, et al. Use of therapeutic drug monitoring to characterize cefepime-related neurotoxicity. Pharmacotherapy. 2023;43(1):6-14. doi:10.1002/phar.2744
- FDA Drug Safety Communication: Cefepime and risk of seizure in patients not receiving dosage adjustments for kidney impairment. U.S. Food and Drug Administration. Published January 19, 2016. Accessed August 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-cefepime-and-risk-seizure-patients-not-receiving-dosage-adjustments.
- Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013;17(6):R264. doi:10.1186/cc13094
- Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing Cefepime Neurotoxicity: A Systematic Review. Open Forum Infect Dis. 2017;4(4):ofx170. doi:10.1093/ofid/ofx170
- Maan G, Keitoku K, Kimura N, et al. Cefepime-induced neurotoxicity: systematic review. J Antimicrob Chemother. 2022;77(11):2908-2921. doi:10.1093/jac/dkac271
- Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276. Published 2017 Nov 14. doi:10.1186/s13054-017-1856-1
- Qian ET, Casey JD, Wright A, et al. Cefepime vs Piperacillin-Tazobactam in Adults Hospitalized With Acute Infection: The ACORN Randomized Clinical Trial. JAMA. 2023;330(16):1557-1567. doi:10.1001/jama.2023.20583
- Chow KM, Szeto CC, Hui AC, Wong TY, Li PK. Retrospective review of neurotoxicity induced by cefepime and ceftazidime. Pharmacotherapy. 2003;23(3):369-373. doi:10.1592/phco.23.3.369.32100
- Boschung-Pasquier L, Atkinson A, Kastner LK, et al. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect. 2020;26(3):333-339. doi:10.1016/j.cmi.2019.06.028
- Martínez-Rodríguez JE, Barriga FJ, Santamaria J, et al. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am J Med. 2001;111(2):115-119. doi:10.1016/s0002-9343(01)00767-7
- Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant. 2008;23(3):966-970. doi:10.1093/ndt/gfm713
- Rhoney DH, Tam VH, Parker D Jr, McKinnon PS, Coplin WM. Disposition of cefepime in the central nervous system of patients with external ventricular drains. Pharmacotherapy. 2003;23(3):310-314. doi:10.1592/phco.23.3.310.32108
- Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008;42(12):1843-1850. doi:10.1345/aph.1L307
- Fernández-Fernández FJ, Ameneiros-Lago E. Cefepime-Induced Encephalopathy: A Possible Additional Mechanism of Neurotoxicity. Neurocrit Care. 2020;32(2):641. doi:10.1007/s12028-019-00894-2
- Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314. doi:10.1016/j.kint.2023.10.018
- Kim MC, Kim SO, Kim SH, et al. Efficacy and Safety of Cystatin C-Guided Renal Dose Adjustment of Cefepime Treatment in Hospitalized Patients with Pneumonia. J Clin Med. 2020;9(9):2803. doi:10.3390/jcm9092803
- Honore PM, Spapen HD. Cefepime-induced neurotoxicity in critically ill patients undergoing continuous renal replacement therapy: beware of dose reduction!. Crit Care. 2015;19:455. doi:10.1186/s13054-015-1179-z
- Cusumano JA, Klinker KP, Huttner A, Luther MK, Roberts JA, LaPlante KL. Towards precision medicine: Therapeutic drug monitoring-guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity. Am J Health Syst Pharm. 2020;77(14):1104-1112. doi:10.1093/ajhp/zxaa128
- Huwyler T, Lenggenhager L, Abbas M, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017;23(7):454-459. doi:10.1016/j.cmi.2017.01.005