Brain-Computer Interfaces in Neurocritical Care: The Layer 7 Cortical Interface
Published on: August 15, 2024
Neurocritical care relies on the continuous, real-time measurement of numerous physiologic parameters, increasingly capable of driving and interpreting large volumes of data. There is emerging interest in the use of high-resolution, high-channel-count brain computer interfaces for extra-operative monitoring of patients in the neurocritical care unit recovering from an acute injury such as a severe traumatic brain injury (TBI), intracranial hemorrhage, or stroke. This is due to increasing recognition that the brain’s response to such injury can trigger abnormal electrical activity including non-epileptic seizure-like activity and cortical spreading depolarizations, which are both prognostically important and potentially treatable.
Patients may experience subtle changes in cortical activity that precede observable symptoms and are only detectable at high resolutions, potentially providing clinicians with a valuable window of opportunity for intervention. In addition, abnormal electrical activity at high resolution may follow distinct patterns that can only be recognized by advanced analytics and machine learning algorithms.
Furthermore, such patients often suffer impaired or fluctuating consciousness, making it difficult to monitor and predict the likelihood of recovery of key neurological functions including motor, speech, and cognition. Brain computer interfaces (BCI) represent an attractive option to both monitor patients for abnormal electrical activity and dynamically assess residual cortical function in patients unable to comply with standard forms of neurologic assessment.
As the field of brain computer interfaces has evolved, approaches to this technology have become roughly divided into those utilizing high bandwidth but tissue damaging electrode designs and those utilizing low bandwidth but minimally invasive or non-invasive implantation approaches. But for BCI to reach its potential, a different approach may be needed, according to Dr. Ben Rapoport, co-founder and Chief Science Officer of Precision Neuroscience. “[BCI] must be able to achieve high bandwidth data exchange while minimizing the amount of damage to brain tissue caused by device placement and use. This led to the founding of Precision Neuroscience, where we leveraged advances in thin film technology to create the Layer 7 Cortical Interface, a new type of cortical surface array.”
Thin-film-technology involves printing a layer of microelectrodes on a flexible thin polymer that allows higher density of electrodes, maximizing interface bandwidth and scalability while minimizing direct damage to the cortical surface. The Layer 7 is designed to operate as a “micro-electrocorticography” array with platinum leads and electrodes insulated and mechanically supported by polyimide. To provide a sense of scale, each electrode is designed to be about as large as a single neuron. The electrodes contact the cortical surface in a conformal manner in the subdural space, gently hugging the brain’s surface. The Layer 7 system provides approximately 625 times the spatial resolution compared to an AdTech 1x4 surface electrode, one of the standard existing intraoperative recording arrays (Figure 1).
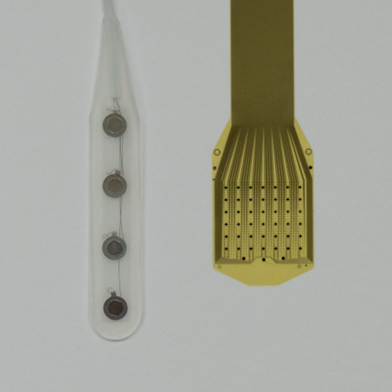
Figure 1: Side-by-side comparison between an AdTech 4-contact electrode strip and the Layer 7 Cortical Interface. The Layer 7 device contains 1,024 electrodes in ~1.5 cm2 of surface array, with an inter-electrode spacing of 400 µm. This provides an electrode to surface area ratio of 683 electrode per cm. By comparison, the standard AdTech strip electrodes typically contain 4-8 electrodes with 1 cm2 spacing between each electrode. For a standard 1 x 4 electrode strip, this provides an electrode to surface area ratio of 0.91 electrodes per cm2.
High-density intraoperative cortical surface recordings have so far been performed in 17 patients as proof-of-concept studies of the Layer 7 Cortical Interface at West Virginia University Rockefeller Neuroscience Institute, Mount Sinai Health System in New York, and Perelman School of Medicine at the University of Pennsylvania (Penn Medicine). The Institute has partnered with Precision Neuroscience to test the Layer 7 device during cranial procedures where intraoperative cortical mapping is routinely performed. “Reproduction of signal detection in this study using this high-density array demonstrates the potential impact of this technology in both intraoperative monitoring and the neurocritical care and epilepsy monitoring units to enable physicians to identify zones of interest and functional boundaries more quickly and confidently and with greater precision than is currently possible,” said Dr. Rapoport. “High-resolution electrocorticography also opens the door to more precise diagnostic and prognostic tools and techniques in neurocritical care.”
This study recruited from neurosurgical patients who were undergoing a craniotomy that included brain mapping as part of the normal surgical procedure and consented for participation in this exploratory study. Led by principal investigator Dr. Peter Konrad and lead surgeon Dr. Sanjay Bhatia, the study team first performed standard brain mapping, then placed the Layer 7 electrode array over the same region as the standard grid electrodes that were previously used. Four patients underwent awake language mapping and three patients underwent asleep motor mapping, with 1024-channel electrocortical recordings obtained while patients conducted simple counting tasks (in the language mapping cases) or while the contralateral median nerve was stimulated (in the motor mapping cases) (Figure 2).
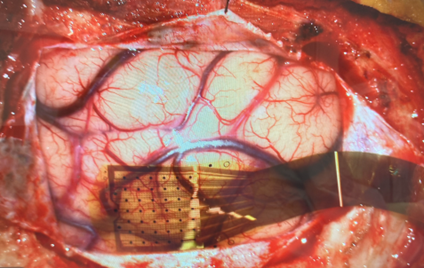
Figure 2: Layer 7 Cortical Interface placed on brain to obtain intraoperative recording.
Preliminary results of this study demonstrated the ability of the system to safely acquire, process, and display high-spatiotemporal-resolution electrocortical data in real time. The device enabled submillimeter resolution functional mapping, which can help guide surgeons perform safer and faster surgeries. Study investigators were pleased with the ease of use of the device and excited about the potential improvements to the clinical workflow that the device could enable, which are planned to be formally assessed in upcoming studies. “This is a remarkable achievement in real-time detection of electrical brain activity mapped with such high resolution,” said Peter Konrad, the study’s principal investigator. “It’s as if I was seeing the patient’s brain think.”
Further studies of the Layer 7 Cortical Interface will aim to collect robust data in a variety of clinical contexts, including applications in both intraoperative monitoring and the neurocritical care and epilepsy monitoring units. Precision Neuroscience has partnered with additional institutions and is currently in the initial phases of studies with the University of Pennsylvania and Icahn School of Medicine at Mount Sinai. Thus far, these studies have enrolled an additional ten patients combined, with results to be announced at upcoming conferences.